Nitrogen contains 7 protons, 7 neutrons, and 7 electrons.How many protons does Nitrogen have? Nitrogen has 7 protons. How many valence electrons does Nitrogen have? Nitrogen has 5 valence protons. Is Nitrogen reactive or stable? How do you know? Nitrogen is reactive because it needs 8 electrons to have its 2 shells filled. Nitrogen only has 7 electrons.One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. This element is found in group 15 and period 2 of the Periodic Table of the Elements. It has an atomic weight of 14.007 amu. Nitrogen is the seventh element on the periodic table.Even mass nuclei composed of odd numbers of protons and neutrons have integral spins. Examples are I = 1 ( 2 H, 14 N ). Even mass nuclei composed of even numbers of protons and neutrons have zero spin ( I = 0 ). Examples are 12 C, and 16 O.Also Know, how many protons does fluorine have? 9 . In this manner, how many electrons does fluorine have? So for the element of FLUORINE, you already know that the atomic number tells you the number of electrons.That means there are 9 electrons in a fluorine atom. Looking at the picture, you can see there are two electrons in shell one and seven in shell two.
Atoms and Bonding - CAK Flashcards | Quizlet
You know that nitrogen-14 has 7 protonsin the nucleus because it is an isotope of nitrogen, which has an atomic number equal to 7. What is the proton number of nitrogen with an atomic number of 7? Nitrogenhas an atomic number of 7(Z=7) because it has 7 protonsin its nucleus. Some nitrogen atomshave an atomic mass numberof 15 (A=15).In this video we'll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Nitrogen. From the Periodic Ta...Nitrogen has an atomic number of 7. So for an atom to be known as a nitrogen atom, it must have 7 protons. For example, if an atom has 8 protons, it will no longer be nitrogen, but will instead be oxygen. Any atom that doesn't have 7 protons is not nitrogen.The element nitrogen has the atomic number 7 and an atomic mass of 14. How many neutrons does an atom of nitrogen contain? 7 Explanation: From the periodic table, nitrogen has a netron of 7 while its counterpart as well i. e proton and electron
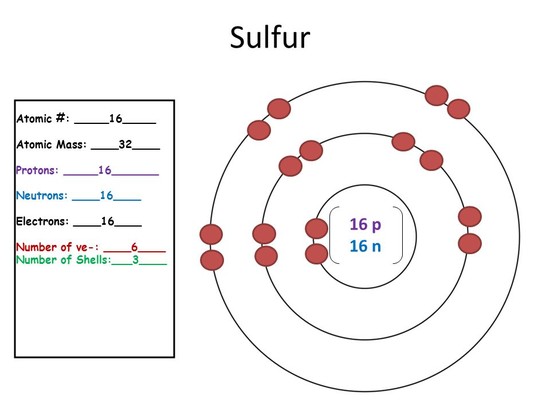
How Many Protons, Neutrons and Electrons Are in a Nitrogen
A nitrogen atom has seven protons in its nucleus. This answer is true for every nitrogen atom in existence, regardless of what isotope of nitrogen it... See full answer below. Become a member and...Has a tiny mass that is practically zero. 10. How many protons does an atom of beryllium have? Beryllium as 4 protons. 11. How many neutrons does an atom of nitrogen have? Nitrogen has a mass of 14 and 7 protons, so 14-7=7 neutrons 12. How many neutrons are in an atom of cesium? Cesium has a mass of 133 and 55 protons, so 133-55= 78 neutrons. 13.From periodic table,we observe that this number for nitrogen is 7.This number is called atomic number and expresses the number of protons in the nitrogen's element.So there are seven protons in nitrogen.The Atomic Number Of Nitrogen Is 7. How Many Protons Does Each Of The Isotopes Have. This problem has been solved! See the answer. nitrogen has three isotopes, 13N, 14N, 15N. the atomic number of nitrogen is 7. how many protons does each of the isotopes have. Expert Answer 100% (1 rating) Previous question Next questionFor example every helium atom has 2 protons, but naturally it can have 1 or 2 neutrons. Also, usually one form is much more abundant than the other, with helium 99.999% is in the 2 neutron form. The number of electrons in an atom can also vary, but if you take a pure sample of the element under normal conditions the number of electrons is equal
Is nitrogen a metal?
The first part in the group is the nonmetal nitrogen (N), adopted via phosphorus (P), another nonmetal. Arsenic (As) (Figure beneath) and antimony (Sb) are the metalloids in this staff, and bismuth (Bi) is a metal. All team 15 elements are solids, with the exception of for nitrogen, which is a gas.
【2 Steps】How Many Protons Does Nitrogen Have?||Number of ...

How many protons are in sulfur 36?

What is nitrogen? Atoms, Elements, Chemistry - Quatr.us ...

How many electrons does nitrogen have? - Quora
How many protons does mercury have?

Argon: How Many Protons Does Argon Have
.PNG)
Solved: How Many Protons Does The Nitrogen Variant With At ...

PPT - How many valence electrons does magnesium have ...

Symbols and the atomic English language messages

Selenium | chemical element | Britannica

PPT - 1 st Six Weeks Review PowerPoint Presentation, free ...

1000+ images about Education ~ 2 Biology (Essential ...

【3 Steps】How Many Neutrons Does Nitrogen Have?||Number of ...

How many electrons does nitrogen have? - Quora

Jewish Physics

⚗️How many protons, neutrons, and electrons does the atom ...

An atom has 6 electrons in its outer shell How many ...

Atoms

How many protons are there in any te atom?
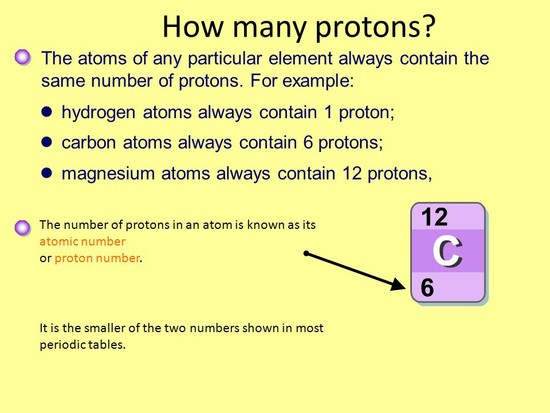
How many protons are in sulfur 36?

Nitrogen Periodic Table Protons Neutrons Electrons ...

0 comments:
Post a Comment